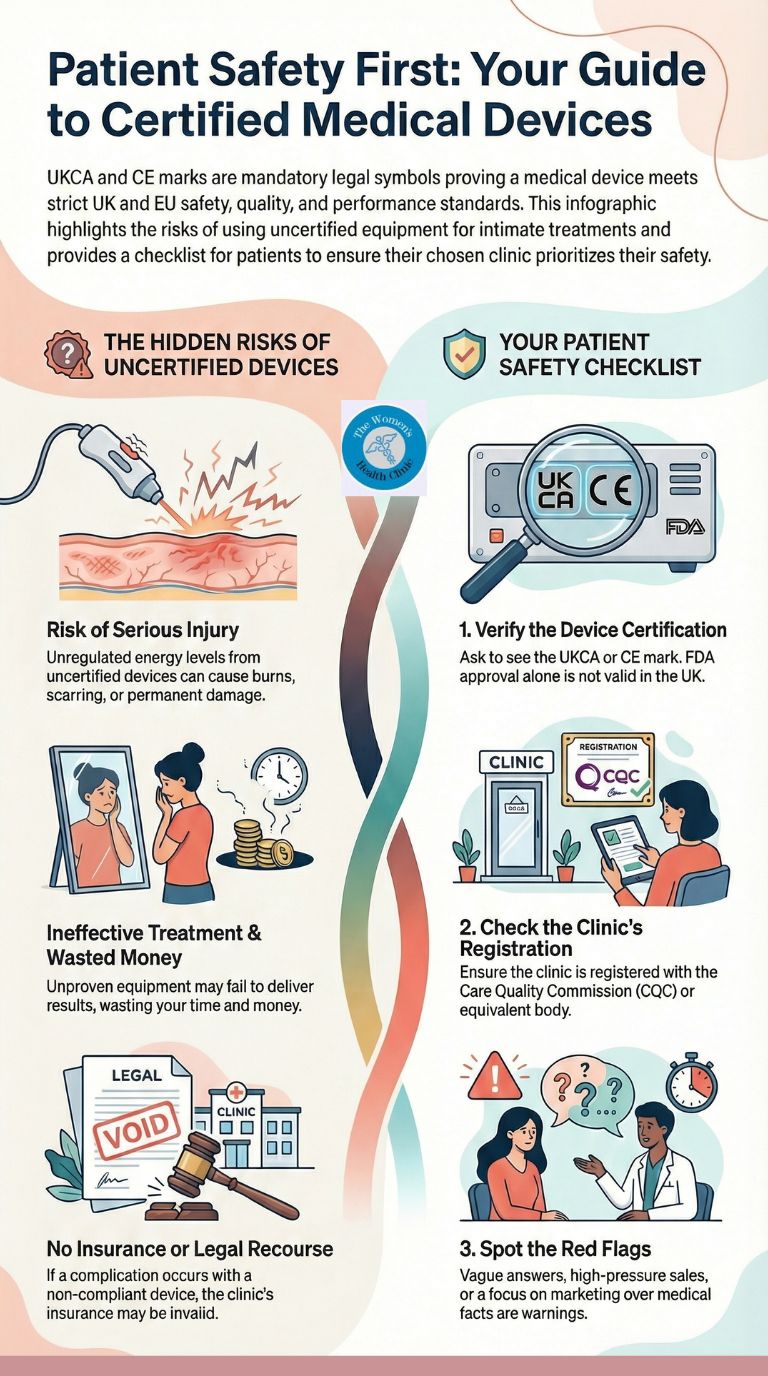
Are devices and products UKCA/CE-marked—why does that matter?
UKCA (UK Conformity Assessed) and CE (Conformité Européenne) marks are mandatory regulatory symbols showing that medical devices meet strict safety, performance, and quality standards. These marks mean the device has been independently assessed, undergoes ongoing surveillance, and can be legally used in clinical practice—protecting you from unproven or dangerous equipment. For intimate treatments like vaginal wellness procedures, certification ensures the technology has appropriate evidence and safeguards in place.
Show Detailed Answer
When you undergo any medical treatment—especially those using devices like lasers, radiofrequency systems, or injection products—you are trusting that the equipment has been rigorously tested for safety and effectiveness. UKCA and CE marking are not optional marketing badges; they are legal requirements that confirm a device complies with regulatory frameworks designed to protect patients.
Understanding these marks empowers you to ask informed questions before consenting to treatment. It is entirely reasonable to request proof of certification, ask about the device class, and understand what post-market surveillance measures are in place. Clinics using certified devices demonstrate transparency and accountability, which are hallmarks of ethical medical practice.
What UKCA and CE Marks Actually Mean
Both marks indicate that a manufacturer has followed a conformity assessment process, which includes:
- Risk Analysis: Identifying potential harms and implementing design controls to minimise them.
- Clinical Evidence: Demonstrating the device performs as intended, supported by clinical data or trials.
- Quality Management Systems: Following ISO 13485 standards for manufacturing consistency and traceability.
- Post-Market Surveillance: Ongoing monitoring for adverse events, with mandatory reporting to regulatory bodies like the MHRA (Medicines and Healthcare products Regulatory Agency) in the UK.
The CE mark has been the standard across Europe, whilst the UKCA mark became necessary for the UK market following Brexit. Devices can carry both marks during transitional periods.
Why Certification Matters for Vaginal Wellness Devices
Treatments such as vaginal laser therapy, radiofrequency tightening, or regenerative injections use equipment that delivers controlled energy or substances into delicate tissue. Without proper certification, there is risk of:
- Thermal Injury: Unregulated energy levels can cause burns, scarring, or permanent damage to the vaginal wall or surrounding structures.
- Infection: Devices that are not designed with appropriate sterility controls can introduce bacteria or compromise tissue integrity.
- Ineffectiveness: Unproven devices may fail to deliver the promised results, wasting your time, money, and emotional energy.
- Lack of Recourse: If something goes wrong with a non-certified device, there may be no clear route for complaint, compensation, or regulatory action.
Device Classes and Risk Categories
Medical devices are classified by risk level, from Class I (lowest risk, e.g., tongue depressors) to Class III (highest risk, e.g., implantable cardiac devices). Most devices used in vaginal wellness fall into Class IIa or IIb, meaning they require third-party conformity assessment by a “Notified Body”—an independent organisation authorised by regulators to review and certify devices.
Higher-class devices undergo more stringent scrutiny, including clinical trials and detailed technical documentation. You can ask your clinic what class their device holds and who the Notified Body is.
Common Concerns & Myths
“Surely all clinics use certified devices?”
Not always. Some aesthetic or wellness clinics may use imported devices that lack UK or EU certification, or equipment intended for “cosmetic” rather than medical use, which bypasses stricter medical device regulations.
“If a device is expensive, it must be safe.”
Price does not equal safety. Certification is the legal and clinical standard—not cost. Always ask to see the UKCA/CE certificate or manufacturer documentation.
“The clinic said it’s FDA-approved, so that’s fine.”
FDA approval (USA) is valuable but does not replace UKCA or CE marking for use in the UK or EU. A device may hold multiple certifications, but the relevant one for your location must be valid.
Clinical Context
Regulatory certification is the foundation of patient safety in modern medicine. The MHRA oversees medical devices in the UK and has the power to issue safety alerts, recall devices, and prosecute non-compliance. Devices used for vaginal wellness—whether for urinary incontinence, atrophy, or cosmetic tightening—must meet the same regulatory standards as devices used in hospital operating theatres. Choosing a clinic that uses certified equipment ensures you are protected by these frameworks. Educational only. Results vary. Not a cure.
Evidence-Based Approaches
Self-Care & Lifestyle
Whilst you cannot personally verify a device’s certification, you can take informed steps before committing to treatment:
- Ask Direct Questions: Request to see the device’s UKCA/CE certificate, manufacturer documentation, or Notified Body details.
- Research the Device Name: Look up the specific model online to confirm it appears on the MHRA database or manufacturer’s certified product list.
- Check Clinic Registration: Ensure the clinic is registered with the Care Quality Commission (CQC) in England, or equivalent bodies in Scotland, Wales, or Northern Ireland.
- Read Patient Information Leaflets: Certified devices come with detailed instructions for use (IFU) and patient information that should be made available to you.
Medical & Specialist Options
Reputable clinics will proactively display certification information and welcome questions about device safety. Red flags include:
- Vague Answers: If staff cannot tell you the device class, manufacturer, or certification status, consider this a warning sign.
- Marketing Over Medicine: Clinics that focus heavily on before/after photos or celebrity endorsements but provide little technical or regulatory detail may prioritise profit over safety.
- Pressure to Proceed Quickly: Ethical practitioners give you time to review information, ask questions, and seek second opinions.
Before committing to treatment, you can meet the clinical team to discuss device certification and safety protocols. Many patients also wish to book a consultation to review all documentation in person.
C. Red Flags (When to Escalate Concerns)
If you experience unexpected pain, burns, scarring, infection, or other adverse events following a device-based treatment, report it immediately to your clinic and to the MHRA via the Yellow Card Scheme. This helps protect future patients and ensures regulatory oversight.
External Resources:
Educational only. Results vary. Not a cure.
Safety Standard: Yes. All our medical devices carry the UKCA (UK Conformity Assessed) or CE mark. This is not just a sticker; it is a legal proof that the device meets strict safety, efficacy, and quality standards required by the MHRA. We do not use "grey import" or unverified machinery.
Why Certification Matters
Think of these marks as a device's "Passport."
- CE Mark (Conformité Européenne): The gold standard for the European Union. It proves the device meets rigorous EU health and safety laws.
- UKCA Mark: The new post-Brexit standard specific to Great Britain. It ensures compliance with UK regulations managed by the MHRA.
- Why both? During the transition period, valid CE marks are still recognized, but new devices must meet UKCA standards. We ensure all our equipment is compliant with the latest legislation.
Many clinics market devices as "FDA Approved" (US Standard). This is meaningless in the UK.
Why FDA isn't enough
A device can be FDA cleared but fail UK safety standards. If a UK clinic uses a device only because it has FDA approval, they may be operating illegally. Always check for the CE or UKCA mark.
A laser might have a CE mark for skin (dermatology), but that doesn't mean it's safe for the vagina (gynecology).
- Our Promise: We use devices (like the NuV that is similar to MonaLisa Touch or Emsella) that are specifically certified for Gynecological Health. Using a skin laser internally is "off-label" and carries higher risks of burns or scarring.
If a clinic treats you with a non-compliant machine (a "grey import" or cheap copy), their medical insurance will not cover them.
- Your Safety: In the unlikely event of a complication, you need to know the clinic is insured. Using regulated devices ensures full insurance validity and patient protection.